Senior Living Blog
Diabetes: Are you over-monitoring your blood sugar?
Diabetes: Are you over-monitoring your blood sugar? New evidence shows that many people with type 2 diabetes test their blood sugar levels too often, which costs hundreds of dollars a year. Read More… Powered by NewsAPI.org
December 13, 2018

How Does Weight Loss “Fix” Type 2 Diabetes?
A recent trial has shown that weight loss can send type 2 diabetes into remission. Now, researchers explain why it happens, and why it doesn’t always work. Read More… Powered by NewsAPI.org How does weight loss ‘fix’ type 2 diabetes?
August 2, 2018

What You Need To Know About High Blood Pressure
In the United States, approximately 85 million people have high blood pressure – about 1 in every 3 adults over 20, according to the American Heart Association (AHA). The National Institutes of Health (NIH) estimate that about two-thirds of people over the age of 65 in the U.S. have high blood pressure. If left untreated or uncontrolled, high blood […]
July 13, 2018

Abbott’s Prick-Free Glucose Monitor Now Available For Medicare Patients With Diabetes
Medicare patients have gained access to a device that allows people with diabetes to keep tabs on their glucose levels without having to routinely prick their fingers, Abbott Laboratories announced January 4th. Abbott’s FreeStyle Libre System, which was approved by the U.S. Food and Drug Administration in September and launched in U.S. pharmacies in November, […]
January 4, 2018
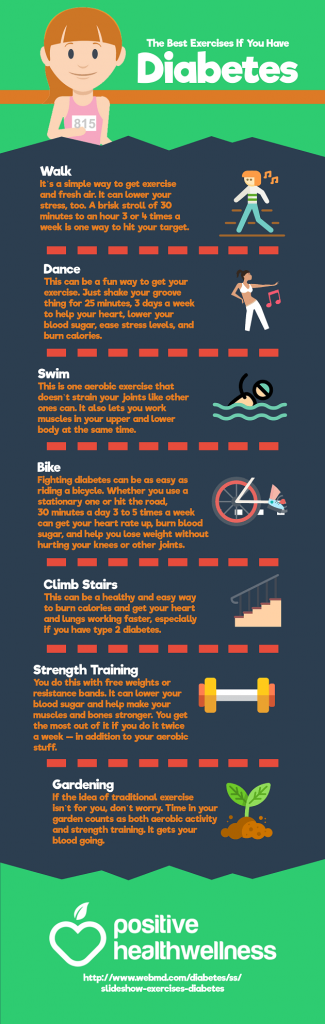
The Best Exercises If You Have Diabetes
This article was originally posted on PositiveHealthWellness.com
November 8, 2017

What Does Medicare Cover for People With Diabetes?
For people with diabetes, Medicare Part B will cover blood glucose monitors, test strips, lancet devices, and lancets. In addition, glucose management solutions for those with diabetes are covered whether someone uses insulin or not.
April 4, 2017

Silent Heart Attacks—Don’t Think They’re Not the Real Thing
Everyone knows the symptoms of a heart attack, right? The feeling of tightness … chest pain … pain in the arms … sweating … shortness of breath ….
April 3, 2013

More on Diabetes and Your Heart
Recently, we discussed the connection between diabetes and heart disease.
February 14, 2013

Dangers, Decisions, and Diabetes
August 4, 2012
How to Keep Your Brain Young
July 27, 2012
